
サンプル調製例(Minimal dyes)
Minimal dyesを使用した2Dパターンおよびサンプル調製例(Lysis bufferの組成、細胞または組織の処理方法、二次元電気泳動のプロトコール)を以下に示します。
Sample Types
- Canorhabditis elegans (C.elegans), pH 3-10 NL IPG strip
- Drosophila melanogaster (D. melanogaster), pH 3-10 NL IPG strip
- Escherichia coli (E.coli) cell culture, pH 3-10 NL IPG strip
- Escherichia coli (E.coli) cell culture, pH 4-5 IPG strip
- Escherichia coli (E.coli) cell culture, pH 4.5-5.5 IPG strip
- Escherichia coli (E.coli) cell culture, pH 4-7 IPG strip
- Escherichia coli (E.coli) cell culture, pH 5-6 IPG strip
- Escherichia coli (E.coli) cell culture, pH 5.5-6.7 IPG strip
- Escherichia coli (E.coli) cell culture, pH 6-9 IPG strip
- Human serum, pH 3-10 NL IPG strip
- Mouse cerebellum, pH 3-10 NL IPG strip, 8 M urea lysis buffer
- Mouse cerebellum, pH 3-10 NL IPG strip, 7 M urea, 2 M thiourea lysis buffer
- Mouse striatum, pH 3-10 NL IPG strip
- Mouse skeletal muscle, pH 3-10 NL IPG strip, 8 M urea lysis buffer
- Mouse skeletal muscle, pH 3-10 NL IPG strip, 7 M urea, 2 M thiourea lysis buffer
- NIH 3T3 fibroblasts, pH 3-10 NL IPG strip
- Rat heart, pH 3-10 NL IPG strip
- Rat liver, pH 3-10 NL IPG strip
- Rat Kidney, pH 3-10 NL IPG strip
- Rat plasma, pH 3-10 NL IPG strip
- Saccharomyces cerevisiae (S. cerevisiae), pH 3-10 NL IPG strip
- Bladder epithelial carcinoma T24 cell line, pH 3-10 NL IPG strip
Canorhabditis elegans (C. elegans), pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
3 mg/ml sample lysate in water.
Sample precipitated using acetone on ice for 1 h.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Supernatant discarded and pellet resuspended in lysis buffer.
Lysate concentration 2.5 mg/ml prior to labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |

25 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Drosophila melanogaster(D. melanogaster), pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
25 mM Tris,
4 % CHAPS
(pH 8.0-8.5). |
Whole flies mechanically homogenized, directly in lysis buffer.
Incubated on ice for 1 h.
Centrifuged at 4 ℃ (12,000×g, 20 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |

50 μg protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Escherichia coli (E. coli) cell culture, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, cathodic cup loading.
50 μA per strip.
1. 500 V, 1 h, step.
2. 1,000 V, 1 h, step.
3. 8,000 V, 8 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Escherichia coli (E.coli) cell culture, pH 4-5 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 4-5 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Escherichia coli (E.coli) cell culture, pH 4.5-5.5 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 4.5-5.5 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Escherichia coli (E.coli) cell culture, pH 4-7 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 4-7 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |
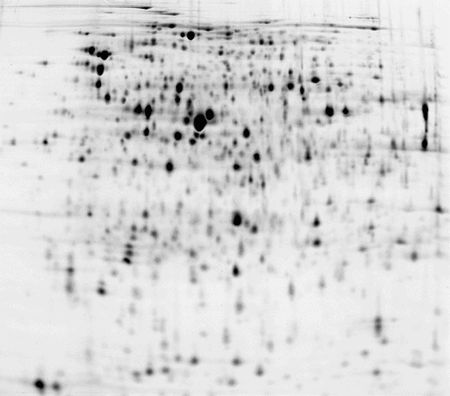
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Escherichia coli (E.coli) cell culture, pH 5-6 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 5-6 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 0,00 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |
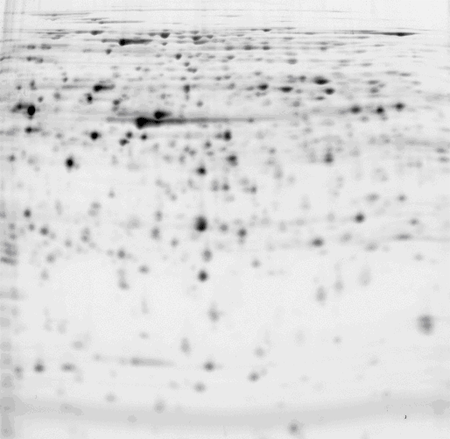
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Escherichia coli (E.coli) cell culture, pH 5.5-6.7 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 5.5-6.7 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Escherichia coli (E.coli) cell culture, pH 6-9 IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Lysis buffer added to cell pellet.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 6-9 Immobiline™ DryStrip.
DeStreak reagent used.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Human serum, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
8 M urea,
40 mM Tris,
4 % CHAPS
(pH 8.0). |
Sample diluted
directly in lysis buffer to 10 mg/ml. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |
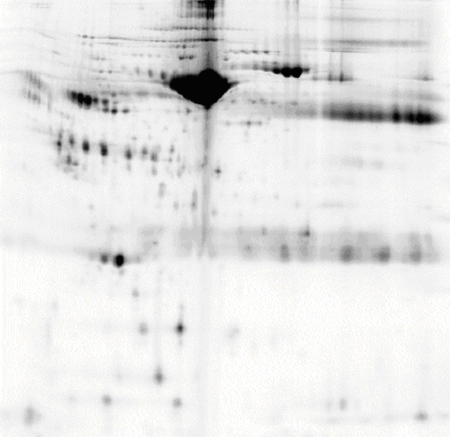
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy3 minimal dye.
Mouse cerebellum, pH 3-10 NL IPG strip, 8 M urea lysis buffer
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
8 M urea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Washed 4 × with saline solution (0.9 %, 10 ml).
Saline solution drained.
Cut into small pieces, lysis buffer added and mechanically homogenized at room temperature.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |
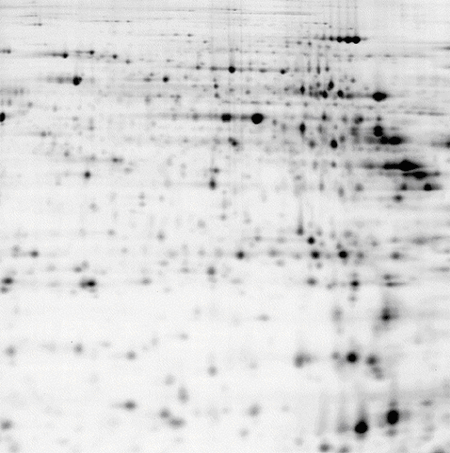
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Mouse cerebellum, pH 3-10 NL IPG strip, 7 M urea, 2 M thiourea lysis buffer
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Washed 4 × with saline solution (0.9 %, 10 ml).
Saline solution drained.
Cut into small pieces, lysis buffer added and mechanically homogenized at room temperature.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye
Mouse striatum, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Mechanically homogenized in ice-cold lysis buffer.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye
Mouse skeletal muscle,
pH 3-10 NL IPG strip, 8 M urea lysis buffer
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
8 M urea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Washed 4 × with saline solution (0.9 %, 10 ml).
Saline solution drained.
Cut into small pieces, lysis buffer added and mechanically homogenized at room temperature.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |
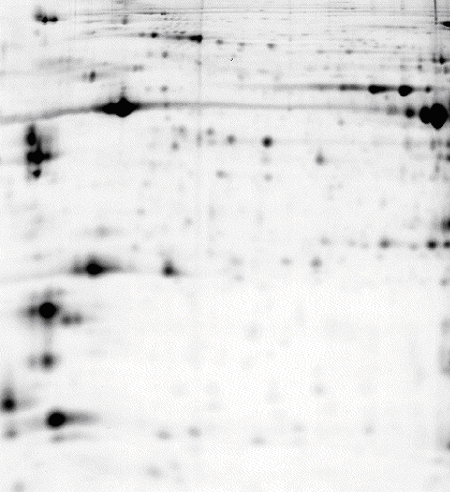
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Mouse skeletal muscle,
pH 3-10 NL IPG strip, 7 M urea, 2 M thiourea lysis buffer
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Washed 4 × with saline solution (0.9 %, 10 ml).
Saline solution drained.
Cut into small pieces.
Lysis buffer added and mechanically homogenized at room temperature.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
NIH 3T3 fibroblasts, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS,
5 mM magnesium
acetate
(pH 8.5). |
Trypsinized cells washed twice with wash buffer and diluted
1 in 10 with lysis buffer.
Sample sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |
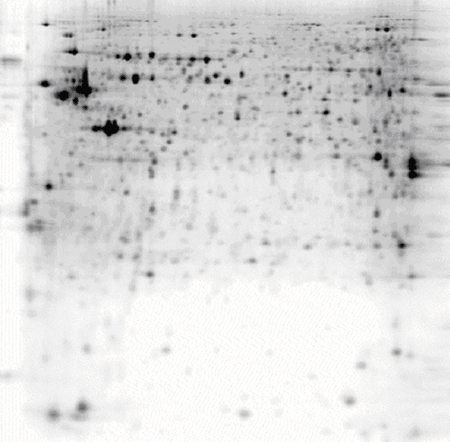
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Rat heart, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
10 mM Tris,
5 mM magnesium acetate,
4 % CHAPS
(pH 8.0). |
1 g of tissue placed in 10 ml of lysis buffer.
Tissue mechanically homogenized and then centrifuged at 10 ℃ (12,000×g, 1 h).
Pellet
discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 4-7 Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Rat liver, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
10 mM Tris,
5 mM magnesium acetate,
4 % CHAPS
(pH 8.0). |
1 g of tissue placed in 10 ml of lysis buffer.
Tissue mechanically homogenized and then centrifuged at 10 ℃ (12,000×g, 1 h).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight. |

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Rat kidney, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
5 mM magnesium acetate,
4 % CHAPS
(pH 8.5). |
Washed 3 × with saline solution and drained.
Cut into small pieces, lysis buffer added and mechanically homogenized at room temperature.
Centrifuged at 4 ℃ (13,000 rpm, 10 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight.
|
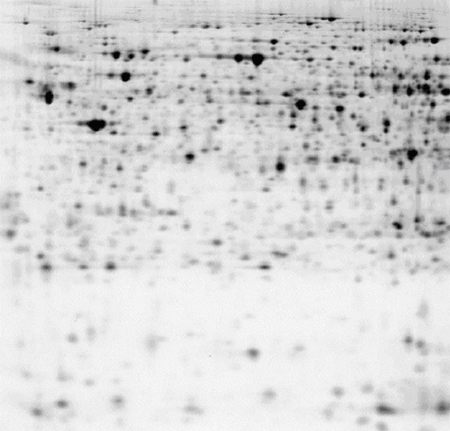
50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Rat plasma, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
5 mM magnesium acetate,
4 % CHAPS
(pH 8.0). |
8 ml of plasma mixed with 10ml of lysis buffer.
Centrifuged at 10 ℃ (12,000×g, 1 h).
Pellet discarded and supernatant used for labelling.
Lysate concentration 10.9 mg/ml prior to labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
2 W per gel overnight.
|

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Saccharomyces cerevisiae (S. cerevisiae), pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
30 mM Tris,
4 % CHAPS
(pH 8.5). |
Dried cell preparation resuspended in lysis buffer.
Sonicated on wet ice with low-intensity 30 s pulses until the lysate turned clear.
Centrifuged at 4 ℃ (12,000×g, 5 min).
Pellet discarded and supernatant used for labelling. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight.
|

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
Bladder epithelial carcinoma T24 cell line, pH 3-10 NL IPG strip
| Lysis buffer |
Method of cell or tissue disruption |
1st and 2nd dimension protocols |
7 M urea,
2 M thiourea,
10 mM Tris,
5 mM magnesium acetate,
4 % CHAPS
(pH 8.0). |
Medium was removed, cells washed twice in PBS
and scraped from the flasks.
Cells centrifuged and pellets washed twice in wash buffer
(10 mM Tris, pH 8, 5 mM magnesium acetate).
Pellets resuspended in lysis buffer. |
1st Dimension
24 cm, pH 3-10 NL Immobiline™ DryStrip.
Ettan™ IPGphor™ IEF unit, anodic cup loading.
50 μA per strip.
1. 300 V, 3 h, step.
2. 600 V, 3 h, gradient.
3. 1,000 V, 3 h, gradient.
4. 8,000 V, 3 h, gradient.
5. 8,000 V, 4 h, step.
2nd Dimension
12.5 % Ettan™ DALTtwelve
1.5 W per gel overnight.
|

50 μg of protein labelled with CyDye™ DIGE Fluor, Cy5 minimal dye.
お問合せフォーム
※日本ポールの他事業部取扱い製品(例: 食品・飲料、半導体、化学/石油/ガス )はこちらより各事業部へお問い合わせください。
お問い合わせありがとうございます。
後ほど担当者よりご連絡させていただきます。
|
























